Can Research Subject to Subpart B and That Includes
Interventions or invasive procedures to the woman or the fetus or involving. Subpart B is found in 45 CFR 46 DHHS.
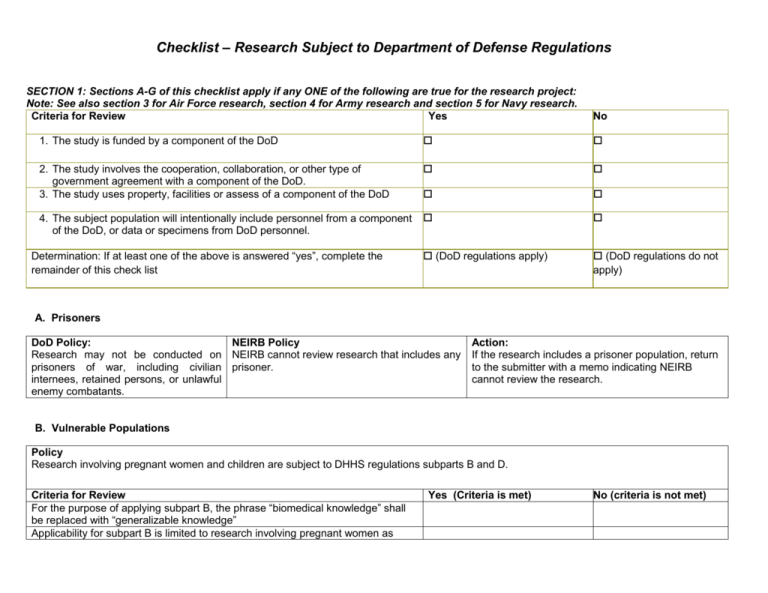
Checklist Research Subject To Department Of Defense Regulations
For DHHS-funded research 45 CFR subpart B applies to all research involving pregnant women.
. 46207 Research not otherwise approvable which presents an opportunity to understand prevent or alleviate a serious problem affecting the health or welfare of pregnant women fetuses or neonates. Other Dangerous Atmospheres in Shipyard Employment. DoD research involving pregnant women fetuses neonates must comply with.
The Common Rule includes additional protections for certain vulnerable research subjects. For children as defined in Sec. A Except as provided in paragraph b of this section this subpart applies to all research involving pregnant women human fetuses neonates of uncertain viability or nonviable neonates conducted or supported by the Department of Health and Human Services DHHS.
Subpart B The Final Rule changes the pre-2018 rule to allow the exemptions to apply to Subpart C for research involving a broader subject population if the research only incidentally includes prisoners. This term refers to the regulations which apply to research involving pregnant women and fetuses as subjects. Fetuses or neonates as participants.
Subpart B - Informed Consent of Human Subjects Sec. As participants in research that is more than minimal risk and included. Yes each of the exemptions in 45 CFR 46104 may be applied to research subject to Subpart B.
46204 d For research that holds out the prospect of direct benefit to the pregnant woman the prospect of direct benefit both to the pregnant woman and fetus or no prospect of benefit for the woman nor the fetus when risk to the fetus is not greater than minimal and the purpose of the research is provide important. 1 Subpart B. This includes all research conducted in DHHS facilities by any person and all research conducted in any facility by DHHS employees.
A research study will compare a new combined behavioral and pharmacologic treatment with the standard behavioral treatment alone for individuals. Paragraphs d2i and of this section only may apply to research subject to subpart D involving educational tests or the observation of public behavior when the investigators do not participate in the activities being observed. IRB Approval under Subpart B.
B The exemptions at 46101 b 1 through 6 are applicable to this subpart. 46402a who are pregnant assent and permission are obtained in accord with the provisions of the Protections for Children Involved as Subjects Subpart D. This includes all research conducted in DHHS facilities by any person and all research conducted in any.
For research conducted or supported by DHHS or other federal agencies under Subpart B 45CFR46201-206 the regulations require additional safeguards for their protection in human research. Regarding the reasonably foreseeable impact of the research on the fetus or neonate. The applicability of Subpart B is limited to research involving pregnant women.
505a each fiscal year shall be expended by the State for research development and technology transfer activities. Where scientifically appropriate pre-clinical studies including studies on. When conducting research subject to 45 CFR 46 Subpart B eg involving pregnant women human fetuses and neonates Principal Investigators PIsLead Site Investigators must ensure that the protocol and the performance of the research is in compliance with all applicable sections of Subpart B and the other applicable Subparts of 45 CFR 46 as described in Section.
23 CFR 420107 SPR Subpart B. Reference to State or local laws in this subpart and in 46101 f is. When conducting research subject to 45 CFR 46 Subpart B eg involving pregnant women human fetuses and neonates Principal Investigators PIsLead Site Investigators must ensure that the protocol and the performance of the research is in compliance with all applicable sections of Subpart B and the other.
DHHS Regulations are provided in 45 CFR Part 46. 46205 Research involving neonates. According to 45 CFR subpart B pregnant women or fetuses may be involved in research funded by DHHS if all of the following conditions are met.
RESEARCH INVOLVING PREGNANT WOMEN FETUSES. Encompasses the period of time from implantation until delivery. The Federal cost share is 80 percent unless the Secretary determines that the interests of the Federal-aid.
5020 General requirements for informed consent. E If the research holds out the prospect of direct benefit solely to the fetus then the consent of the pregnant woman and the father is obtained in accord with the informed consent provisions of subpart A of this part except that the fathers consent need not be obtained if he is unable to consent because of unavailability incompetence or. A woman shall be assumed to be pregnant if.
The Final Rule permits the. Except as provided in 5023 and 5024 no investigator may involve a human being as a subject in research covered by these regulations unless the investigator has obtained the legally effective informed consent of the subject or the subject. Through its IRB-Flex policy the University opts to suspend Subpart B and apply equivalent protections for minimal risk research involving PG WomenFetuses.
Can be applied to research subject to Subpart B. There are different kinds of research. Not less than 25 percent of the funds set aside by 23 USC.
46206 Research involving after delivery the placenta the dead fetus or fetal material. Can research subject to subpart b and that includes pregnant women as subjects be exempt from the regulations per 45 cfr 46 if all of the conditions of the exemption are met. Subpart B provides additional protections for pregnant women in vitro fertilization and fetuses Subpart C contains additional protections for prisoners Subpart D does the same for children.
YES research subject to subpart b and that includes pregnant women as subjects be exempt from the regulations per 45 cfr 46 if all of the conditions of the exemption are met. 29 CFR Part 1915 Subpart B Confined and Enclosed Spaces and. This instruction provides current policy inspection procedures information and guidance to ensure uniform enforcement of the 29 CFR Part 1915 Subpart B standard which became effective on October 24.
Paragraph d2iii of this section may not be applied to research subject to subpart D. Can research subject to subpart b and that includes pregnant women as subjects be exempt from the regulations per 45. Each of the exemptions at this section may be applied to research subject to subpart B if the conditions of the exemption are met.
C The provisions of 46101 c through i are applicable to this subpart. The exemptions at this section do not apply to research subject to subpart C except for research aimed at involving a broader subject population that only incidentally includes prisoners.

The Code Of Federal Regulations 45 Cfr 46 The Nih Follows All Subparts Of The Hhs Regulations A Basic Hhs Policy 4 Protection Human Services Coding Lesson
Comments
Post a Comment